DAD-311
DIAPHORASE from Microorganism
PREPARATION and SPECIFICATION
| Appearance | Yellowish amorphous powder, lyophilized | |
|---|---|---|
| Activity | GradeⅢ 500 U/mg-solid or more | |
| Contaminants | Myokinase | ≤5.0×10-1% |
| NADH oxidase | ≤1.0×10-1% | |
PROPERTIES
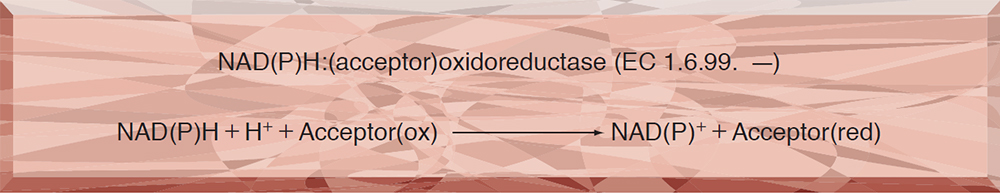
| Stability | Stable at −20℃(Fig.1) |
|---|---|
| Molecular weight(Gel-filtration) | approx. 48,000 |
| Michaelis constant | 2.2×10-4M(NADH),2.9×10-2M(NADPH) |
| Inhibitors | Fe3+, Mn2+, Cu2+, Pb2+ |
| Isoelectric point | 5.0 |
| Optimum pH | 8.0(Fig.3) |
| Optimum temperature | 60℃(Fig.4) |
| pH Stability | 5.0−10.0(Fig.5) |
| Thermal stability | below 70℃(Fig.6) |
| Substrate specificty | Either NADH or NADPH can be used as a reductant. |
| Effect of various chemicals | (Table 1) |
APPLICATIONS
This enzyme is useful for colorimetric determination of NAD(P)H and many dehydrogenases when coupled with various dyes which act as hydrogen acceptors from NAD(P)H.
ASSAY
Principle

The reduction of DCPIP(2,6-dichlorophenol-indophenol) is measured at 600 nm by spectrophotometry.
Unit definition
One unit causes the decrease of one micromole of DCPIP per minute under the conditions described below.
Method
Reagents
| A. Buffer solution | 0.2M Tris-HCl, pH 8.0 |
|---|---|
| B. NADH solution | 36mM (Prepare freshly and store on ice) |
| C. DCPIP solution | 2.4mM[7.8mg DCPIP(Mw:326.11)/10ml of H2O](Should be prepared fresh) |
| D. Enzyme diluent | Buffer solution (A) containing 0.5% of Tween20 |
Procedure
1.Prepare the following reaction mixture in a cuvette (d=1.0cm) and equilibrate at 37℃ for about 4 minutes.
| 2.4ml | H2O | |
|---|---|---|
| 0.3ml | Buffer solution | (A) |
| 0.1ml | NADH solution | (B) |
| Concentration in assay mixture | |
|---|---|
| Tris buffer | 27 mM |
| NADH | 1.2 mM |
| DCPIP | 80 μM |
| Tween20 | ca.167μg/ml |
2.Add 0.1 ml of the enzyme solution* and mix by gentle pipetting and equilibrate at 37℃ for another 1 min.
3.Add 0.1 ml of DCPIP solution (C) and mix by rapid inversion.
4.Record the decrease of optical density at 600 nm against water for 3 to 4 min in a spectrophotometer thermostated at 37℃, and calculate the ΔOD per minute from the initial linear portion of the curve (OD test).
At the same time , measure the blank rate (OD blank) by the same method as test except that the enzyme diluent is added instead of the enzyme solution.
*Dissolve the enzyme preparation in ice-cold buffer solution (A) (approx. 1.0% solution), dilute to 0.10−0.25U/ml with ice-cold enzyme diluent (D) and store on ice.
Calculation
Activity can be calculated by using the following formula :
Volume activity (U/ml) =
-
ΔOD/min (OD test−OD blank)×Vt×df
20.9×1.0×Vs
= ΔOD/min×1.43×df
Weight activity (U/mg) = (U/ml)×1/C
| Vt | : Total volume (3.0ml) |
| Vs | : Sample volume (0.1ml) |
| 20.9 | : Millimolar extinction coefficient of DCPIP under the assay conditions (cm2/micromole) |
| 1.0 | : Light path length (cm) |
| df | : Dilution factor |
| C | : Enzyme concentration in dissolution (c mg/ml) |
Table 1. Effect of Various Chemicals on Diaphorase
[The enzyme dissolved in 0.1M HEPES buffer, pH7.5 (5U/ml) was incubated with each chemical at 25℃ for 1hr.]
-
Chemical Concn.(mM) Residual activity(%) None ー 100 Metal salt 2.0 MgCl2 102 CaCl2 99 Ba(OAc)2 100 FeCl3 4.4 CoCl2 94 MnCl2 55 ZnCl2 84 Cd(OAc)2 101 NiCl2 101 CuSO4 23 Pb(OAc)2 46 AgNO3 94 MIA 1.0 104 NaF 2.0 105 -
Chemical Concn.(mM) Residual activity(%) NaN3 2.0 104 EDTA 5.0 105 o-Phenanthroline 2.0 105 α,α′-Dipyridyl 1.0 102 Borate 5.0 104 IAA 2.0 105 NEM 2.0 106 Hydroxylamine 2.0 107 TritonX-100 0.10% 109 Brij 35 0.10% 109 Tween 20 0.10% 116 Span 20 0.10% 113 Na-Cholate 0.10% 110 SDS 0.05% 91 DAC 0.05% 110
-
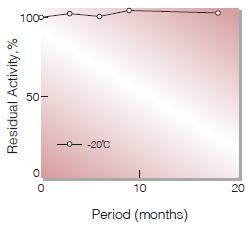
Fig.1. Stability (Powder form)
(kept under dry conditions)
-
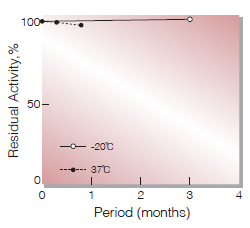
Fig.2. Stability (Powder form)
(Kept under dry conditions)
-
Fig.3. pH-Activity
37℃, in 0.1M buffer solution;〇――〇, KPB; △――△, Tris-HCl;□――□, Gly-NaOH
-
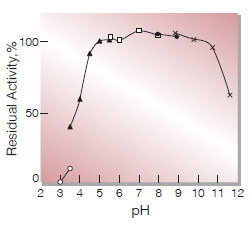
Fig.4. pH-Stability
25℃, 20hr-treatment with 0.1M buffer solution; 〇――〇, Glycine-HCl;▲――▲, Acetate; □――□, KPB;●――●, Tris-HCl; ×――×, Glycine-NaOH
-
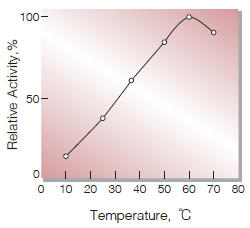
Fig.5. Temperature activity
(in 50mM K-Phoshate buffer, pH7.5)
-
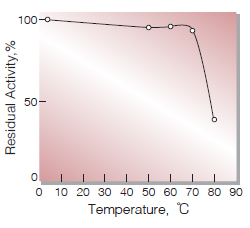
Fig.6. Thermal stability
15 min-treatment with 0.1M K-Phosphate buffer, pH7.5 Enzyme concentration : 10 U/ml
活性測定法(Japanese)
1. 原理

DCPIP(2,6-dichlorophenol-indophenol)の還元量を600nmの吸光度の変化で測定する。
2.定義
下記条件下で1分間に600nmの吸光度を1.0減少させる酵素量を1単位(U)とする。
3.試薬
- 0.2M Tris-HCl緩衝液, pH8.0
- 36mM NADH水溶液(用時調製,氷冷保存)
- 2.4mM DCPIP水溶液〔7.8mgのDCPIP(Mw:326.11)を10mlの蒸留水で溶解する〕(用時調製)
酵素溶液:酵素標品を予め氷冷した蒸留水で溶解し,分析直前に酵素希釈液(D)で0.10〜0.25U/mlに希釈する。
4.手順
1.下記反応液をキュベット(d=1.0cm)に採り,25℃で約5分間予備加温する。
| 2.4ml | 蒸留水 | |
| 0.3ml | 0.2M Tris-HCl緩衝液、pH 8.0 | (試薬A) |
| 0.1ml | 36mM NADH水溶液 | (試薬B) |
2.酵素溶液0.1mlを加えてピペッティングによる混和後,さらに約1分間予備加温する。
3.DCPIP水溶液(試薬C)を0.1mlを加えて,速やかに転倒混和した後,水を対照に37℃に制御された分光光度計で600nmの吸光度変化を3〜4分間記録し,その初期直線部分より1分間当たりの吸光度変化を求める(ODtest)。
4.盲検は1の反応混液に酵素希釈液(0.5%のTween20を含む試薬A),DCPIP水溶液各0.1mlを加え,上記同様に操作を行って1分間当たりの吸光度変化量を求める(ODblank)。
5.計算式
U/ml =
-
ΔOD/min (OD test−OD blank)×3.0(ml)×希釈倍率
20.9×1.0×0.10(ml)
| = ΔOD/min×1.43×希釈倍率 | |
| U/mg | =U/ml×1/C |
| 20.9 | : DCPIPのミリモル分子吸光係数 (cm2/micromole) |
| 1.0 | : 光路長(cm) |
| C | : 溶解時の酵素濃度(c mg/ml) |
CONTACT
お問い合わせ-
各種製品に関するご質問・ご相談はこちらよりお問い合わせください。
