-
IVH-101
INVERTASE from Candida sp.
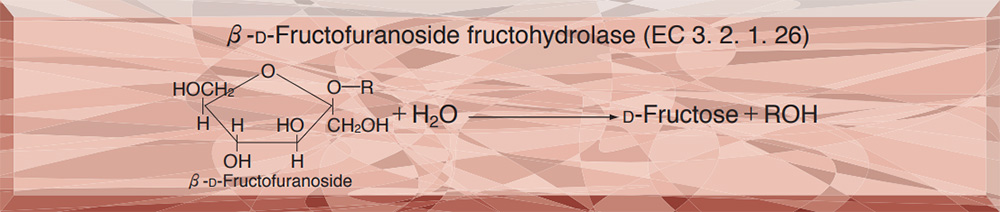
PREPARATION and SPECIFICATION
| Appearance | White amorphous powder, lyophilized | |
|---|---|---|
| Activity | GradeⅠ 100U/mg-solid or more (containing approx. 70% of stabilizer) |
|
| Stabilizer | KH2PO4 | |
PROPERTIES
| Stability | Stable at −20℃ for at least one year(Fig.1) |
|---|---|
| Molecular weight | approx. 260,000 |
| Michaelis constant | 1.5×10-2M (Saccharose) |
| Structure | Glycoprotein containing approx. 50% of carbohydrates |
| Optimum pH | 3.5−4.0(Fig.3) |
| Optimum temperature | 60−70℃(Fig.4) |
| pH Stability | pH 4.0−6.0 (50℃, 10min)(Fig.5) |
| Thermal stability | below 60℃ (pH 4.5, 10min)(Fig.6) |
| Substrate specificity | This enzyme hydrolyzes saccharose and raffinose, but does not hydrolyze inulin and melezitose. 6) |
APPLICATIONS
This enzyme is useful for enzymatic determination of saccharose and for the structure investigation of carbohydrates containing β-D-fructofuranoside residue.
ASSAY
Principle

The appearance of reducing sugars is measured by the modified Fehling-Lehmann-Schoorl method. 7)
Unit definition
One unit causes the formation of one milligram of reducing sugars equivalent to the glucose at 3 minutes under the conditions described below (This activity is equivalent to the international unit that hydrolyzes one micromole of saccharose per minute at the same temperature).
Method
Reagents
| A. Saccharose solution | 5.0%[5.0g saccharose/100ml of 80mM acetate buffer, pH 4.5 (add 2−3 drops of toluene for preservation)] | |
|---|---|---|
| B. Alkaline solution | (103g NaOH, 346g Rochelle salt・4H2O/1,000ml of H2O) | |
| C. CuSO4 solution | 6.93% (69.3g CuSO4・5H2O/1,000ml of H2O) | |
| D. KI solution | 30% (300g KI/1,000ml of H2O)(Store in a brownish bottle) | |
| E. H2SO4 solution | 25% | |
| F. Na2S2O3 solution | 50mM (49.638g Na2S2O3・5H2O, 4.0g Na2CO3 (stabilizer)/4,000ml of H2O)(Store in a brownish bottle and keep for 3−4 days before use) | |
| G. Soluble starch solution | 2.0% (Dissolve by boiling) (Should be prepared fresh) | |
| H. Enzyme diluent | 50mM acetate buffer, pH 4.5 | |
Procedure
1. Pipette 1.0ml of substrate solution (A) into a test tube and equilibrate at 20℃ for about 5 minutes.
2. Add 1.0ml of the enzyme solution (pre-incubated at 20℃) and mix.
| Concentration in assay mixture | |
|---|---|
| Acetate buffer | 65mM |
| Saccharose | 73mM |
3. After exactly 3 minutes at 20℃, add 2.0ml of alkaline solution (B) to stop the reaction.
At the same time, prepare the blank by first mixing the substrate solution with 2.0ml of alkaline solution after a 3 min-incubation at 20℃, followed by the addition of the enzyme solution and carry out the same procedure as the test (Procedure 4−8).
4. Transfer the stopped reaction mixture from the test tube to a 100ml volume of Erlenmeyer flask. Rinse the inside of the test tube with about 3ml of distilled water and transfer the rinsings to the flask. Repeat this procedure three times.
5. Add 2.0ml of CuSO4 Solution (C) and place the flask directly on a electrothermic heater (1.2 KWH) in the presence of a glass bead (5mmφ) to prevent bumping.
6. Keep for exactly 2 minutes in a boiling state and cool down to room temperature under running water.
7. Add 2.0ml each of KI solution (D) and H2SO4 solution (E) in this order.
8. Shake the flask and determine the amount of residual Cu++ by titration with Na2S2O3 solution (F) in the presence of a few drops of soluble starch (G) as an indicator.
9. Record the titers (ml) of the test (t) and the blank (b), and calculate the titration difference (Δtiter, ml).
*Dissolve the enzyme preparation in ice-cold distilled water and dilute to 2.0−9.0 U/ml with the enzyme dilute (H), immediately before assay.
Calculation
Activity can be calculated by using the following formula :
Volume activity (U/ml) =
-
Δtiter (b−t)×F×df
0.600×Vs
= Δtiter×F×1.66×df
Weight activity (U/mg) = (U/ml)×1/C
| Vs | : Sample volume (1.0ml) |
| 0.600 | : Titration difference (ml) of 50mM Na2S2O3 solution (F=1.00) for 1.0mg of reducing sugar (glucose) |
| F | : Concentration factor of 50mM Na2S2O3 (F should be determined by the iodotimetry method in each time of the preparation). |
| C | : Enzyme concentration in dissolution (c mg/ml) |
REFERENCES
1)H.Negoro; J.Agric.Chem.Soc. (Japan), 31, 253 (1957).
2)K.Myrbck; The Enzyme (2nd ed.) Vol. 14, p.379 (1960), AP.
3)T.Kaya; J.Agr.Chem.Soc. (Japan), 38, 417 (1964).
4)J.Hoshino and A.Momose; J.Gen.Appl.Microbiol., 12, 163 (1966).
5)O.Lampen et el.; J.Biol.Chem., 243, 1573 (1968).
6)H.Negoro; J.Ferment, Technol., 51, 879, (1973).
7)T.Yamamoto, J.Kumada and T.Sawai; Bull.Agric.Biol.Chem.Soc. (Japan); 21, 185 (1957).
-
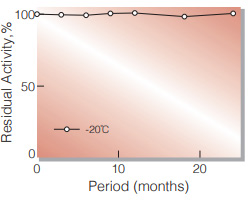
Fig.1. Stability (Powder form)
(kept under dry conditions)
-
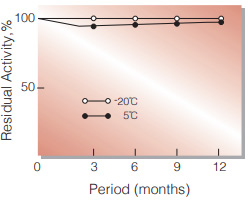
Fig.2. Stability (Powder form)
(kept under dry conditions)
-
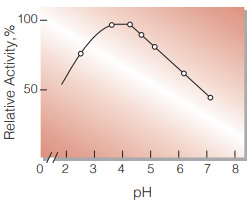
Fig.3. pH-Activity
20℃, 3min-reaction in the following buffer solution:pH2〜3,0.1M glycineHCI; pH4〜5,50mM acetate ; pH6〜7,50mM phosphate
-
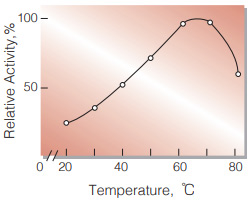
Fig.4. Temperature activity
(3min-reaction in 50mM acetate buffer, pH4.5)
-
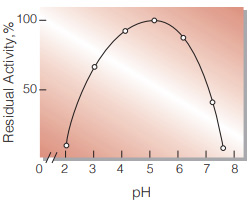
Fig.5. pH-Stability
50℃, 10min-treatment with the following buffer solution: pH2〜3,0.1M glycine-HCI; pH4〜5,50mM acetate; pH6〜8,50mM phosphate
-
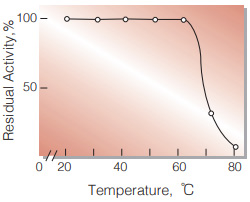
Fig.6. Thermal stability
(10min-treatment with 50mM ace tatebuffer, pH4.5)
活性測定法(Japanese)
1. 原理

還元糖の生成量をFehling-Lehman-Schoorl変法で測定する。
2.定義
下記条件下で3分間に1.0mgのグルコース相当還元糖を生成する酵素量を1単位(U)とする。
3.試薬
- 5.0%サッカロース溶液(5.0gのサッカロースを80mM酢酸緩衝液,pH4.5に溶解し100mlとする(防腐の為にトルエン2〜3滴を滴下しておく))
- ロッシェル塩アルカリ溶液(103gのNaOH及び346gの酒石酸カリウム・ナトリウム塩・4H2Oを蒸留水に溶解し,1,000mlとする)
- 6.93%硫酸銅溶液(69.3gのCuSO4・5H2Oを蒸留水に溶解し,1,000mlとする)
- 30%ヨウ化カリウム溶液(300gのKIを蒸留水に溶解し,1,000mlとする)(褐色瓶中で保存)
- 25%硫酸溶液
- 50mMチオ硫酸ナトリウム溶液(49.638gのNa2S2O3・5H2O及び4.0gのNa2CO3を蒸留水に溶解し,4,000mlとする)(褐色瓶中で保存,調製後3〜4日放置して使用する)
- 2.0%可溶性でんぷん溶液(2.0gの可溶性でんぷんを蒸留水100mlに懸濁し煮沸溶解する。指示薬として使用)(用時調製)
酵素溶液:酵素標品を予め氷冷した蒸留水で溶解し,分析直前に50mM酢酸緩衝液,pH 4.5で2.0〜9.0 U/mlに希釈する。
4.手順
1.試験管に基質溶液(A)1.0mlを採り,20℃で約5分間予備加温する。
2.予め20℃に調温されていた酵素溶液1.0mlを加え,反応を開始する。
3.20℃で正確に3分間反応させた後,ロッシェル塩アルカリ溶液(B) 2.0ml加えて反応を停止させる。
4.反応停止後の混液を約100ml容三角フラスコへ移し,更に試験管を約3mlの蒸留水で3回洗浄して三角フラスコへ回収する。
5.硫酸銅溶液(C)を2.0ml加え,突沸防止の為に直径5mmφのガラス玉1ケを入れて電気ヒーターで加熱する。
6.沸騰状態で正確に2分間保ったのち,流水中で室温迄冷却する。
7.ヨウ化カリウム溶液(D) 2.0ml及び硫酸溶液(E)2.0mlをこの順序に加え,よく混和した後50mMチオ硫酸ナトリウム溶液(F)で滴定する。(指示薬として2〜3滴の可溶性デンプン液(G)を加えておく)(tml)。
8.盲検は基質溶液(A) 1.0mlを20℃で3分間放置後,ロッシェル塩アルカリ溶液(B)を加えて混和し,次いで酵素溶液1.0mlを加えて調製する。以下手順④〜⑦を操作して滴定値を求める(b ml)。
5.計算式
U/ml =
-
滴定値の差 (b ml−t ml)×F×希釈倍率
0.600×1.0(ml)
| = 滴定値の差×F×1.66×希釈倍率 | |
| U/mg | = U/ml×1/C |
| 0.600 | : 還元糖(グルコース)1.0mgに相当して生ずる50mMチオ硫酸ナトリウム溶液(F=1.00)の滴定値差(ml/mg) |
| F | : 50mMチオ硫酸ナトリウム溶液の濃度補正係数 |
| C | : 溶解時の酵素濃度(c mg/ml) |
CONTACT
お問い合わせ-
各種製品に関するご質問・ご相談はこちらよりお問い合わせください。
