-
GYK-301
GLYCEROL KINASE from Cellulomonas sp.
PREPARATION and SPECIFICATION
| Appearance | White amorphous powder, lyophilized | |
|---|---|---|
| Activity | GradeⅢ 20U/mg-solid or more (containing approx. 50% of stabilizers) |
|
| Contaminants | NADH oxidase | ≤1.0×10-2% |
| Catalase | ≤1.0×10-1% | |
| Phosphatase (pH 6.0) | ≤2.0×10-3% | |
PROPERTIES
| Stability | Stable at −20℃ for at least one year(Fig.1) |
|---|---|
| Molecular weight | approx. 128,000 (by gel filtration) |
| Isoelectric point | 4.2 |
| Michaelis constants | 4.4×10-5M (Glycerol), 4.3×10-4M (ATP) |
| Inhibitors | p-Chloromercuribenzoate, heavy metal ions (Pb++, Fe++, Hg++, Ag+) |
| Optimum pH | 9.8 (G-3-PDH system), 7.8 (G-3-P oxidase system)(Fig.4) |
| Optimum temperature | 50℃(Fig.5) |
| pH Stability | pH 5.5−10.0 (25℃, 20hr)(Fig.6) |
| Thermal stability | below 40℃ (pH 7.5, 15min)(Fig.7) |
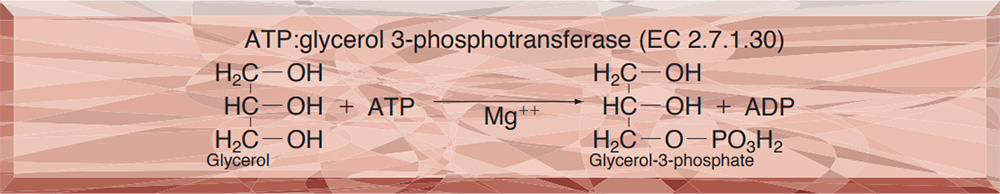
| Substrate specificity | This enzyme catalyzes the stereospecific transfer of the terminal phosphoryl moiety of ATP to one of the primary hydroxyl group of glycerol, forming sn-glycerol-3-P. The enzyme has the highest specificity for glycerol, and also phosphorylates dihydroxyacetone and glyceraldehyde (Table 1,2). Mg++ is essentially required for the reaction. |
| Effect of various chemicals | (Table 3) |
APPLICATIONS
This enzyme is useful for enzymatic determination of glycerol and triglyceride when coupled with glycerol-3-phosphate oxidase (=G-3-P oxidase, G3O-321) or pyruvate kinase and lactate dehydrogenase (LCD-209, LCD-211, LCD-221), lipoprotein lipase (LPL-311, LPL-314) in clinical analysis.
ASSAY
Principle


The appearance of NADH is measured at 340nm by spectrophotometry.
Unit definition
One unit causes the formation of one micromole of NADH per minute under the conditions described below.
Method
Reagents
| A. Glycine-hydrazine buffer, pH 9.8 | 0.2M[Weigh 1.5g of glycine and 20.8g of hydrazine hydrate (MW=50.06), dissolve in 70ml of H2O, add 0.4ml of 1.0M MgCl2 and after adjusting the pH to 9.8 with 2.0N HCl or 2.0N KOH, fill up to 100ml with H2O] | |
|---|---|---|
| B. Glycerol solution | 0.1M (Should be prepared fresh) | |
| C. ATP solution | 0.1M (Should be prepared fresh) | |
| D. NAD+ solution | 14mM (Should be prepared fresh) | |
| E. Glycerol-3-phosphate dehydrogenase (G-3-PDH) solution | Crystalline suspension in 3.2M ammonium sulfate solution (10mg/ml, ca. 60 U/mg from Roche) | |
| F. Enzyme diluent | 20mM K-phosphate buffer pH 7.5 containing 0.2% BSA | |
Procedure
1. Prepare the following reaction mixture in a cuvette (d=1.0cm) and equilibrate at 25℃ for about 5 minutes.
| 3.0 ml | 0.2M Glycine-hydrazine buffer, pH 9.8 | (A) |
| 0.10ml | Substrate solution | (B) |
| 0.05ml | ATP solution | (C) |
| 0.10ml | NAD+ solution | (D) |
| 0.03ml | G-3-PDH solution | (E) |
| Concentration in assay mixture | |
|---|---|
| Glycine buffer | 0.18 M |
| Glycerol | 3.0 mM |
| ATP | 1.5 mM |
| NAD+ | 0.42mM |
| MgCl2 | 3.6 mM |
| G-3-P DH | ca.5.4 U/ml |
2. Add 0.05ml of the enzyme solution* and mix by gentle inversion.
3. Record the increase in optical density at 340nm against water for 3 to 4 minutes in a spectrophotometer thermostated at 25℃ and calculate the ΔOD per minute from the initial linear portion of the curve (ΔOD test).
At the same time, measure the blank rate (ΔOD blank) by using the same method as the test except that the enzyme diluent is added instead of the enzyme solution.
*Dissolve the enzyme preparation in ice-cold enzyme diluent (F), dilute to 0.2−0.4U/ml with the same buffer and store on ice.
Calculation
Activity can be calculated by using the following formula :
Volume activity (U/ml) =
-
ΔOD/min (ΔOD test−ΔOD blank)×Vt×df
6.22×1.0×Vs
= ΔOD/min×10.7×df
Weight activity (U/mg) = (U/ml)×1/C
| Vt | : Total volume (3.33ml) |
| Vs | : Sample volume (0.05ml) |
| 6.22 | : Millimolar extinction coefficient of NADH (cm2/micromole) |
| 1.0 | : Light path length (cm) |
| df | : Dilution factor |
| C | : Enzyme concentration in dissolution (c mg/ml) |
REFERENCES
1) Methods of Enzymatic Analysis, vol. 1, p.468 (H.U.Bergmeyer ed., 2nd English Edition), Verlag Chemie Weinheim & Academic Press, New York-London (1974).
2) S.Hayashi and E.C.C.Lin.; J.Biol.Chem., 212, 1030 (1967).
Table 1. Substrate Specificity of Glycerol kinase (Pyruvate kinase-Lactate dehydrogenase system2), pH 7.5)
[Pyruvate kinase-Lactate dehydrogenase system with 50mM HEPES buffer, pH 7.9]
-
Substrate (6mM) Relative activity(%) Glycerol 100 Glycerol-α-monochlorohydrin 0.09 Ethylene glycol - 1,2-Propanediol - 1,3-Propanediol 0.07 1,3-Butanediol - 1,4-Butanediol - -
Substrate (6mM) Relative activity(%) 2,3-Butanediol - D-Mannitol - D-Sorbitol - D-Glucose - Ribitol - Methanol - Ethanol -
-, Not detected
Table 2. Values of Km and Vmax for Various Substrate (Pyruvate kinase-Lactate dehydrogenase system2), pH 7.5)
-
Substrate Km (M) Vmax(Relative value) Glycerol 4.4×10-5* 100 Dihydroxyacetone 6.0×10-3 152 D,L-Glyceraldehyde 5.8×10-3 76
*This value was taken from that determined by G-3-PDH system, pH 9.8.
Table 3. Effect of Various Chemicals on Glycerol kinase
[The enzyme dissolved in 0.1M acetate buffer, pH 6.0 (40U/ml) was incubated at 30℃ for 1hr with each chemical.]
-
Chemical Concrn.(mM) Residual
activity(%)None - 100 Metal salt 2.0 MgCl2 100 CaCl2 100 Ba(OAc)2 100 FeCl3 75 CoCl2 100 MnCl2 100 Zn(OAc)2 99 NiCl2 98 CuSO4 100 Pb(OAc)2 88 AgNO3 0 HgCl2 0 -
Chemical Concrn.(mM) Residual
activity(%)PCMB 2.0 22 MIA 2.0 96 NaF 2.0 97 NaN3 20 97 EDTA 5.0 101 o-Phenanthroline 2.0 96 α,α′-Dipyridyl 2.0 92 Borate 50 100 Triton X-100 0.1% 99 Na-cholate 0.1% 97
Ac, CH3CO; PCMB, p-Chloromercuribenzoate; MIA, Monoiodoacetate.
-
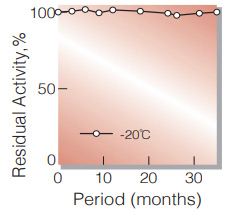
Fig.1. Stability (Powder form)
(kept under dry conditions)
-
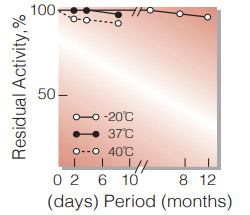
Fig.2. Stability (Powder form)
(kept under dry conditions)
-
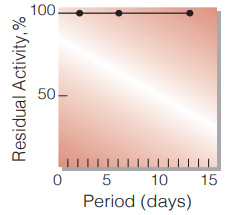
Fig.3. Stability (Liquid form at 37℃)
(enzyme concetration: 400-500 U/ml buffer composition:50mM K-phosphate buffer, contg. 3.2M ammonium sulfate, pH6.3)
-
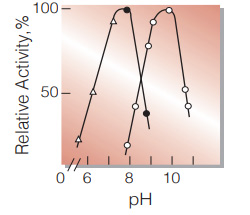
Fig.4. pH-Activity
(25℃; G-3-PDH system(○̶̶○,0.18M glycine-hydrazine buffer); G-3-P oxidasesystem(●̶̶● ,50mM Tris-HCI butfer; △̶̶△,50mM(2-N-Morpholino)ethanesulfonic acid-NaOH buffer.))
-
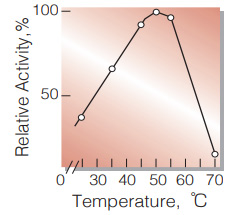
Fig.5. Temperature activity
(in 0.18M glycine-hydrazine buffer, pH9.8)
-
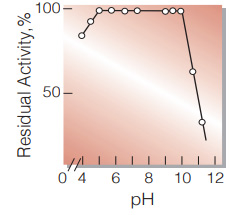
Fig.6. pH-Stability
(25℃; 20hr-treatment with 50mM buffer solution: pH4.0-6.0, acetate; pH6.0-9.0, K-phosphate; pH9.0-11.0,K2CO3-NaHCO3)
-
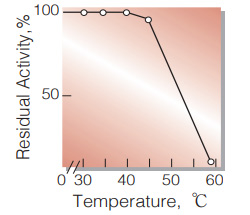
Fig.7. Thermal stability
(15min-treatment with 50mM K-phosphate buffer, pH7.5)
活性測定法(Japanese)
1. 原理


NADHの生成量を340nmの吸光度の変化で測定する。
2.定義
下記条件下で1分間に1マイクロモルのNADHを生成する酵素量を1単位 (U)とする。
3.試薬
- 0.2Mグリシン-ヒドラジン緩衝液,pH9.8(グリシン1.5gおよびヒドラジン(抱水)20.8gを蒸留水80mlに溶解後,1.0M MgCl20.4mlを添加し,2N HClあるいは2N KOHでpHを9.8に調整,次いで最終液量を蒸留水で100mlとする)
- 0.1M グリセロール水溶液 (用時調製)
- 0.1M ATP水溶液 (用時調製)
- 14mM NAD+水溶液 (用時調製)
- グリセロール-3-リン酸脱水素酵素(G-3-PDH)溶液 〔Roche製硫安懸濁結晶酵素 (10mg/ml,約60U/mg)〕
酵素溶液:酵素標品を予め氷冷した0.2%BSAを含む20mM K-リン酸緩衝液,pH 7.5で溶解し,同緩衝液で0.2〜0.4U/mlに希釈して氷冷保存する。
4.手順
1.下記反応混液をキュベット(d=1.0cm)に調製し,25℃で約5分間予備加温する
| 3.0ml | グリシン-ヒドラジン緩衝液 | (A) |
| 0.10ml | 基質溶液 | (B) |
| 0.05ml | ATP水溶液 | (C) |
| 0.10ml | NAD+水溶液 | (D) |
| 0.03ml | G-3-PDH溶液 | (E) |
2.酵素溶液0.05mlを添加し,ゆるやかに混和後,水を対照に25℃に制御された分光光度計で340nmの吸光度変化を3〜4分間記録し,その初期直線部分から1分間当りの吸光度変化を求める(ΔOD test)。
3.盲検は反応混液①に酵素溶液の代りに酵素希釈液(20mM K-リン酸緩衝液,pH 7.5)を0.05ml加え,上記同様に操作を行って,1分間当りの吸光度変化を求める(ΔODblank)。
5.計算式
U/ml =
-
ΔOD/min (ΔOD test−ΔOD blank)×3.33(ml)×希釈倍率
6.22×1.0×0.05(ml)
| = ΔOD/min×10.7×希釈倍率 | |
| U/mg | = U/ml×1/C |
| 6.22 | : NADHのミリモル分子吸光係数(cm2/micromole) |
| 1.0 | : 光路長(cm) |
| C | : 溶解時の酵素濃度(c mg/ml) |
CONTACT
お問い合わせ-
各種製品に関するご質問・ご相談はこちらよりお問い合わせください。
