LPP-229
ALKALINE PHOSPHATASE from Microorganism
PREPARATION and SPECIFICATION
| Appearance | Transparent liquid | |
|---|---|---|
| Activity | GradeⅡ 30,000U/ml or more | |
| Contaminants | Adenosine deaminase | ≤ 1.0×10-4% |
| Phosphodiesterase | ≤ 3.0×10-3% | |
PROPERTIES
| Stability | Stable at 4℃ |
|---|---|
| Molecular weight | approx. 104,000 |
| Optimum pH | 9.5(Fig. 1) |
| Optimum temperature | ≧ 60℃ (Fig. 2) |
| pH Stability | pH 5.5-10.4 (25℃, 16hr)(Fig. 3) |
| Thermal stability | below 65℃ (pH 7.0, 60min) (Fig. 4) |
APPLICATIONS
This enzyme is useful for molecular biology.
ASSAY
Principle
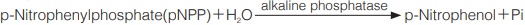
The appearance of p-Nitrophenol is measured at 405nm by spectrophotometry.
Unit definition
One unit causes the formation of one micromole of p-Nitrophenol per minute under the conditions described below.
Method
Reagents
| A. Diethanolamine buffer | 1M[Dilute 9.66ml of diethanolamine (MW=105.14) in 60ml of H2O, add 5ml of 0.1M MgCl2 and, after adjusting the pH to 9.8 with 2N HCl, fill up to 100ml with H2O](Prepare fleshly) |
|---|---|
| B. pNPP solution | 0.674M [2.5g of p-Nitrophenylphosphate disodium salt (MW=371.16) in 10ml Diethanolamine buffer (A)](Prepare fleshly) |
| C. Enzyme diluent | 30mM Triethanolamine, 1mM MgCl2, 0.1mM ZnCl2, 0.5% Sodium cholate, pH7.6 |
Procedure
1.Prepare the following working solution (30.5ml) in a brownish bottle and store on ice (Prepare freshly).
| 30ml | Diethanolamine buffer | (A) |
| 0.5ml | pNPP solution | (B) |
| Concentration in assay mixture | |
|---|---|
| Diethanolamine buffer | 0.97 M |
| p-Nitrophenylphosphate | 11 mM |
| MgCl2 | 4.8 mM |
2.Pipette 3.0ml of working solution into a cuvette (d=1.0cm) and equilibrate at 37℃ for about 5 minutes.
3.Add 0.1ml of the enzyme solution* and mix by gentle inversion.
4.Record the increase in optical density at 405nm against water for 3 to 5 minutes in a spectrophotometer thermostated at 37℃, and calculate the ∆OD per minute from the initial linear portion of the curve (∆OD test).
At the same time, measure the blank rate (∆OD blank) by using the same method as the test except that the enzyme diluent (C) is added instead of the enzyme solution.
*Dilute the enzyme preparation to 0.1-0.3U/ml with ice-cold enzyme diluent (C), immediately before assay.
Calculation
Activity can be calculated by using the following formula:
Volume activity (U/ml) =
-
∆OD/min(∆OD test-∆OD blank)×Vt×df
18.5×1.0×Vs
= ∆OD/min×1.676×df
Weight activity (U/mg) = (U/ml)×1/C
| Vt | : Total volume (3.1ml) |
| Vs | : Sample volume (0.1ml) |
| 18.5 | : Millimolar extinction coefficient of p-Nitrophenol under the assay condition (cm2/micromole) |
| 1.0 | : Light path length (cm) |
| df | : Dilution factor |
-
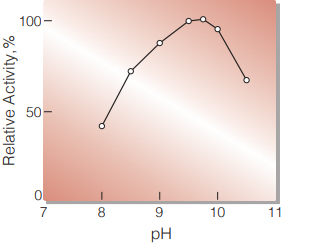
Fig.1 pH-Activity
(in 1M Diethanolamine buffer, pH 8-10.5)
-
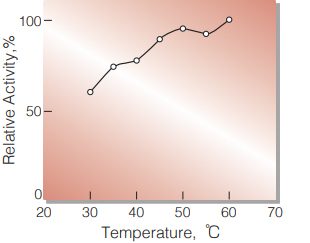
Fig.2 Temperature activity
(in 1M Diethanolamine buffer, pH 10.25 )
-
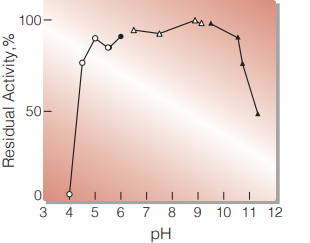
Fig.3 pH-Stability
25℃, 16hr-treatment with 0.1M buffer solution: pH 4-6, dimethylglutaric acid-NaOH; pH 6-8, K-phosphate; pH 8-9, Tris-HCl; pH 9-10, glycine-NaOH. Enzyme concentration: 10U/ml
-
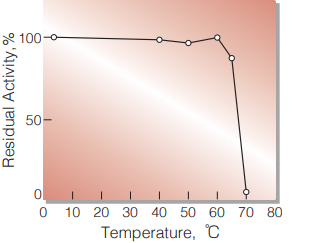
Fig.4 Thermal stability
15min-treatment with 50mM K-phosphate buffer, pH 7.0. Enzyme concentration: 10U/ml
活性測定法(Japanese)
1. 原理
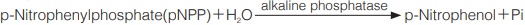
p-Nitrophenolの生成量を405nmにおける吸光度の変化で測定する。
2.定義
下記条件下で1分間に1マイクロモルのp-Nitrophenolを生成する酵素量を1単位(U)とする。
3.試薬
- 1Mジエタノールアミン緩衝液、pH9.8 [9.66mlのジエタノールアミン (MW=105.14)を蒸留水60mlで希釈後、0.1M MgCl2 5mlを添加する。さらに2.0N HClで37℃におけるpHを9.8に調整し、最終液量を100mlとする] (用時調製)
- 0.674M pNPP溶液 [2.5gのp-ニトロフェニルリン酸二ナトリウム塩 (MW=371.16)を10mlの緩衝液Aに溶解する] (用時調製)
- 酵素溶液: 30mM トリエタノールアミン、1mM MgCl2、0.1mM ZnCl2、0.5% Sodium cholate, pH7.6で反応直前に0.1~0.3U/mlに希釈する。
4.手順
1.下記反応混液(30.5ml)を調製する。
| 30ml | ジエタノールアミン緩衝液 | (A) |
| 0.5ml | pNPP溶液 | (B) |
2.3.0mlの反応混液をキュベット(d=1.0cm)に移し、37℃で約5分間予備加温する。
3.酵素溶液0.1mlを添加し、ゆるやかに混和する。
4.水を対照に37℃に制御された分光光度計で405nmの吸光度変化を3~5分間記録し、その初期直線部分から1分間当たりの吸光度変化を求める(∆OD test)。盲検は酵素溶液に代えて酵素希釈液を加え、上記同様に操作を行って1分間当たりの吸光度変化を求める(∆OD blank)。
5.計算式
U/ml =
-
ΔOD/min (ΔOD test - ΔOD blank) × 3.00(ml) × 希釈倍率
18.5 × 1.0 × 0.1 (ml)
| = ∆OD/min × 1.676 × 希釈倍率 | |
| Vt | : 総液量 (3.1ml) |
| Vs | : 試料総量 (0.1ml) |
| 18.5 | : p-Nitrophenolのミリモル分子吸光係数 |
| 1.0 | : 光路長(cm) |
CONTACT
お問い合わせ-
各種製品に関するご質問・ご相談はこちらよりお問い合わせください。
